- hiv壳膜蛋白结构

hiv壳膜蛋白结构
基本介绍
HIV壳膜蛋白结构名为“gp120”。由于“gp120”蛋白在艾滋病病毒入侵人体的机制中发挥着非常重要的作用,因此成为研发艾滋病疫苗的一个重要突破口。
研究进展
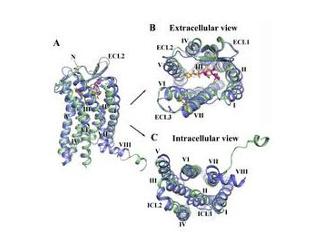 HIV壳膜蛋白结构美国加州理工学院的科学家宣布,他们绘制出了艾滋病病毒(HIV)一种壳膜蛋白的结构图,这使人类在研发艾滋病疫苗的道路上迈出了重要一步。研究成果刊登在线发表于的英国《自然·结构和分子生物学》杂志。参与这项研究的研究员迪斯金说,进一步了解这种蛋白的结构,会使研发艾滋病疫苗的步伐加快。
HIV壳膜蛋白结构美国加州理工学院的科学家宣布,他们绘制出了艾滋病病毒(HIV)一种壳膜蛋白的结构图,这使人类在研发艾滋病疫苗的道路上迈出了重要一步。研究成果刊登在线发表于的英国《自然·结构和分子生物学》杂志。参与这项研究的研究员迪斯金说,进一步了解这种蛋白的结构,会使研发艾滋病疫苗的步伐加快。
Structure of a clade C HIV-1 gp120 bound to CD4 and CD4-induced antibody reveals anti-CD4 polyreactivity
Abstract Strategies to combat HIV-1 require structural knowledge of envelope proteins from viruses in HIV-1 clade C, the most rapidly spreading subtype in the world. We present a crystal structure containing a clade C gp120 envelope. The structure, a complex between gp120, the host receptor CD4 and the CD4-induced antibody 21c, reveals that the 21c epitope involves contacts with gp120, a nonself antigen, and with CD4, an autoantigen. Binding studies using wild-type and mutant CD4 show that 21c Fab binds CD4 in the absence of gp120, and that binding of 21c to clade C and HIV-2 gp120s requires the crystallographically observed 21c-CD4 interaction. Additional binding data suggest a role for the gp120 V1V2 loop in creating a high-affinity, but slow-forming, epitope for 21c after CD4 binds. These results contribute to a molecular understanding of CD4-induced antibodies and provide the first visualization to our knowledge of a potentially autoreactive antibody Fab complexed with both self and nonself antigens.
2015年10月,2015年10月,新型艾滋病疫苗——“全长单链疫苗”首次进行人体试验。“全长单链疫苗”含有HIV的表面蛋白质“gp120”,它们会连接部分的“CD4”接收器。目标是在gp120连接至CD4时(即处于最脆弱的过渡状态时)引发抗体,从而有效地制止它们和第二个接收器“CCR5”连接。

-
最新官图发布/将于年内上市 承载式车身结构/年内正式上市
2025-06-14 19:29:15 查看详情


 求购
求购








